Overview
Brief Summary
Physical Description
Size
Identification Resources
Ecology
Local Distribution and Habitats
Biogeographical Distribution
Micro-habitats and Associations
Crypsis
Life History & Behaviour
Behaviour
Reproduction
Settlement Induction
Evolution & Systematics
Fossil History
Systematics or Phylogenetics
Morphology and Physiology
External Morphology
Internal Anatomy
Molecular Biology & Genetics
Nucleotide Sequences
Molecular Biology
Wikipedia
References
Bibliographies
Names & Taxonomy
Synonyms
Common Names | Comparison of natural cues for the settlement and metamorphosis in Herdmania momus larvae
|
Summary
The biphasic life cycle has been adopted by many species of marine invertebrate and has likely lead to the evolutionary success of many taxonomic groups, such as the ascidians. Herdmania momus is a Pyurid ascidian that has encompasses such a life cycle, and therefore undergoes an important transition from a planktonic larvae to a benthic adult; the following experiment investigates a number of natural settlement cues that may induce this transition. Of the substrates tested, all were effective settlement inductors with one bryozoan genus, the Triphyllozoan, demonstrating consistently higher settlement rates over the experimental period. These results provide some interesting insights into larval settlement and microhabitats of a common and experimentally important Great Barrier Reef ascidian.
|
|
Introduction
A biphasic life cycle – one that begins as a microscopic larva and metamorphoses to a macroscopic adult – is extremely common among invertebrates and has likely lead to the evolutionary success of many species (Rieger 1994). In the marine environment, metamorphosis often involves a transition from planktonic larvae to benthic adult and requires two things. Firstly, larvae must develop competence i.e. the ability to respond to external signals and secondly, presence of a substrate that provides a metamorphosis-inducing cue. The solitary, Pyurid ascidian Herdmania momus is regarded as a good experimental model (Degnan 1991) and a number of settlement cues have previously been investigated. It has been demonstrated that settlement and metamorphosis can be induced naturally using a small, live a sample of the bryozaon Margaretta triplex or chemically, using potassium ions, most effectively as 40mM concentrated potassium chloride (KCl) (Degnan et al., 1997). Conversely, metamorphosis may be inhibited by other substances such as non-geniculate coralline algae (Degnan & Johnson 1999) and allelochemicals produced by sponges (Green et al. 2002). In the absence of a cue (i.e. in fresh sea water alone) H. momus will initiate spontaneous metamorphosis with a development identical to those individuals exposed to known metamorphic cues (Degnan et al., 1997; Degnan 2001).
|
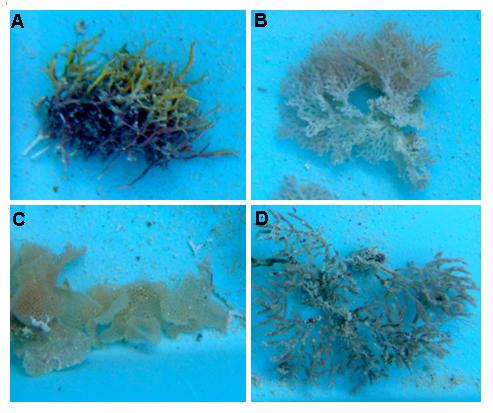
Figure 1. Shows the four natural substrates used. (A) Amphiroa, (B) Reteporella graiffei, (C) Tryphyllozoan, (D) Margaretta Triplex |
|
Previous research has focused largely on chemical inducers (Cloney 1961; Lynch 1961; Patricolo et al.1981; Berking and Hermann 1990) leaving the effects of many potential natural inducers relatively unknown. The following experiment aims to compare the effectiveness of possible natural settlement inducers of H.momus using three species of bryozoan, namely Margaretta Triplex, Tryphyllozoan and Reteporella graiffei as well as the the geniculate coralline algae, Amphiroa. M. Triplex is a known settlement inducer and was expected to induce metamorphosis at rates similar to KCl (the positive control). Although H. momus settlement is inhibited by non-geniculate coralline algae, geniculate coralline algae is an effective settlement inducer of other invertebrate larvae (Williams 2008) and was expected to have a similar effect on H. momus. The effect of R. graiffei and Tryphyllozoan on H.momus settlement is yet to be published but was expected to similar to previously studied bryozoans such as M. Triplex.
|
|
Video shows a dissected gonad of an Herdmania momus specimen releasing sperm
|
Materials and Methods
Specimens of adult H. momus were collected from the underside of numerous coral plates on Heron Reef (23°27’S). Specimens were maintained in flowing, ambient seawater at The University of Queenslands' Heron Island Research Station. For a period of 3 days, adults were kept in under constant light to prevent trickle spawning (Degnan et al. 1996). The gonads of five individuals were dissected, from which the gametes were teased in 0.2µm seawater (FSW) (see video). Fertilisation was initiated by combining all sperm and oocytes in 1.5L of FSW. After 10 minutes the mixture was rinsed using FSW and a 75µm strainer to discard any unwanted debris (Degnan et al. 1996). Developing embryos were removed and maintained in sterile six-well plates at 24°C, each containing 5ml of FSW. Tadpole larvae hatched at about 10 hours after fertilisation.
In order to measure the effectiveness of different inducers, larvae were subject to either FSW (negative control), 40mM KCl (positive control), Margaretta Triplex, Triphyllozaon, Reteporella graeffei, Amphiroa, 4 hours after hatching (expected competency). The number of larvae exhibiting signs of metamorphosis (indicated by a 50% tail resorption) was recorded at 30, 60, 120 and 180 minutes. In all treatments, 3 replicates, each of 30 larvae were used. |
|
Results
Significantly higher percentages of metamorphosed larvae were seen in FSW with Triphylozoan compared to all other treatments. This was consistent throughout the experiment. From 60 minutes onwards, all treatments applied resulted in percentages of larval metamorphosis greater than that of the negative control (FSW) with Triphylozoan consistently producing the highest values (figure 2). M. triplex, KCl, and R. graeffei varied in effectiveness through time whereas FSW, the negative control, had the lowest rates of induction throughout. At 30 minutes after induction cues were introduced, about 50% of larvae with Triphylozoan were beginning to metamorphose whereas about 20% of larvae in KCl and M. triplex, 5% in Amphiroa and 2% R. graiffei were showing similar developmental progression; larvae in FSW were yet to metamorphose at this stage. KCl induced the second highest rates of metamorphosis until 180 minutes when the percentage in R. graiffei rose to 83%. The percentage of larvae metamorphosed in KCl was only significantly higher than others for data recorded at 60 minutes. By 180 minutes, none of the settlement inducers had significantly different percentages of metamorphosed larvae, other than Triphylozoan. All substrates yielded significantly greater induction rates than than FSW with the exception of R. graeffei at 120 minutes (figure 1).
|
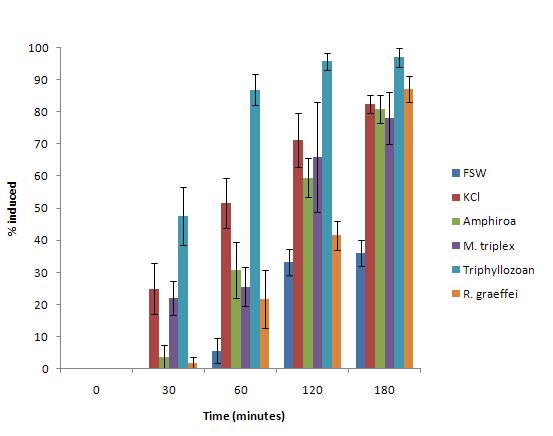
Figure 2. Shows the percent of larvae settled on each substrate over the different 3 hour time period |
|
Discussion
The ability of H. momus larvae to spontaneously settle and metamorphose in FSW alone (Degnan et al. 1997) or when exposed to KCl, a known inducer, allows the determination of other substrum as an inhibitor, inductor or neither. All substrates yielded higher induction rates than FSW, which suggests that all are effective settlement inducers and none are inhibitors. As expected, induction of settlement by M. triplex was similar to KCl for the majority of the experiment, excluding at 60 mintues which may infer different settlement rates or larval variability. Interestingly, Triphylozoan induced the highest rates of metamorphosis which were significantly greater than the positive control for the majority of the experiment. Since potassium ions are known to work on ion channels to induce metamorphosis, and stronger than 40mM KCl is ineffective at producing an enhanced result (Degnan et al. 1997), this may indicate that Triphylozoan either produces a chemical that works on ion channels more efficiently or stimulates and alternative and/or additional induction pathway. This is only a speculative hypothesis however, and further research is needed to aid the elucidation of true mechanisms. Amphiroa and R. graeffei were less effective inducers than KCl for the initial stages of the experiment, suggesting that they may stimulate delayed yet equally as effective induction. These patterns could be further validated by looking at microhabitats of H. momus, focusing on which plants and animals immediately surround them in situ. If the substrates used here are found, this would provide supporting evidence for their role as inductors and their abundance may help explain Herdmania's somewhat sporadic distribution on the reef. Furthermore, if associations vary between species, data would help draw conclusion about whether faster settlement, seen in Triphylozoan, has an effect on ultimate adult habitat.
All substrates tested were able effectively to induce settlement in H. momus larvae with Triphylozoan yielding the highest percentages of larval settlement throughout the experiment. This may have implications for the extent to which these species are found living together on the reef. Although validation of these results in vitro would be advantageous, and the mechanisms by which they occur are yet to be elucidated, these results provide some interesting data on novel larval settlement inducers of the common Great Barrier Reef ascidian, H. momus.

Figure 3. Shows one well of a six well plate containing each treatment. From top left: FSW, KCl, Amphiroa, Margaretta Triplex, Reteporella graeffei, Triphyllozoan
|
|
|